Imaging surveillance after EVAR
Imaging surveillance is necessary in all patients who undergo EVAR to identify complications including endoleak, sac growth, and stent graft migration. A typical surveillance protocol includes CT at 1 month and at 12 months post EVAR. If the 1-month scan shows type 1 or 3 endoleak or unexplained sac increase, an additional scan at 6 months is advisable. If the 12-month scan shows stable sac size with either type 2 or no endoleak, further annual follow-up with duplex is deemed safe. Detection of a new endoleak or sac expansion on duplex ultrasound should prompt further CT evaluation.
Contrast-enhanced US is in conjunction with CTA to assess the flow dynamics of an endoleak, or in unexplained sac expansion with a negative CTA where US microbubbles may demonstrate a slow type 2 endoleak. Similarly, MRI may be used to look for occult endoleaks in cases of unexplained sac expansion although susceptibility artefact from metallic stent grafts and coils may limit image interpretation.
Conventional catheter angiography +/- cone-beam CT may be used as a problem-solving tool when the type and source of an endoleak is inconclusive, for example a type 1 endoleak at the seal zones may be confused with a type 2 endoleak. Rotational catheter angiography with cone-beam CT can be useful in detecting slow-flow type 2 endoleaks in cases of unexplained sac expansion.
Indications for treatment
Type 1 endoleaks represent failure of proximal or distal seal and are associated with elevated sac pressure and an ongoing risk of aneurysm expansion and rupture. They warrant prompt treatment upon detection. Treatment options include aortic cuff, fenestrated cuff, chimney graft, distal stent graft extension and embolization.
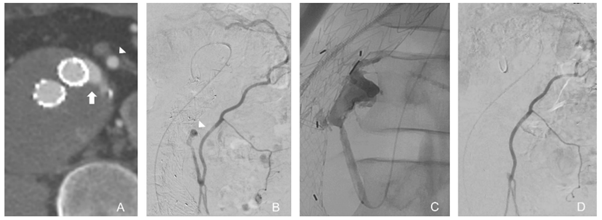
Type 2 endoleaks are caused by retrograde blood flow from aortic branches and are inherently low flow and often transient. They usually involve the IMA and lumbar arteries although accessory renal and internal iliac artery branches may also be involved. They are managed conservatively unless associated with sac expansion. The mainstay of treatment is embolization with a range of embolic agents via multiple routes including transarterial (Figure 1), direct sac puncture or transcaval approaches.
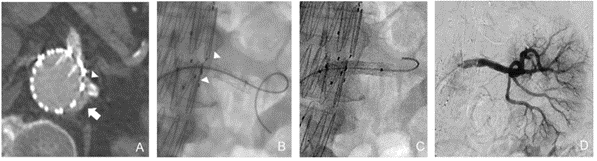
Type 3 endoleaks arise from a structural defect or modular disconnection of stent graft components. This leads to an increase in sac pressure and ongoing risk of aneurysm rupture. They warrant prompt treatment upon detection, usually with additional stent grafts to reline a fabric tear or bridge across a gap (Figure 2).
Type 5 endoleaks represent cases of unexplained sac expansion without an identifiable endoleak. These are challenging to manage and warrant further imaging with multiple modalities to exclude an occult endoleak in the first instance.